Answer: 1 molecule of
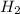 reacts with 1 molecule of
reacts with 1 molecule of
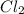 to give 2 molecules of HCl.
to give 2 molecules of HCl.
1 mole of
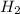 reacts with 1 mole of
reacts with 1 mole of
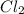 to give 2 moles of HCl.
to give 2 moles of HCl.
Step-by-step explanation:
The given balanced reaction is:
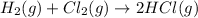
On the particulate level :
1 molecule of
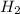 reacts with 1 molecule of
reacts with 1 molecule of
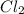 to give 2 molecules of HCl.
to give 2 molecules of HCl.
On molar level:
1 mole of
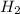 reacts with 1 mole of
reacts with 1 mole of
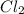 to give 2 moles of HCl.
to give 2 moles of HCl.