Answer: Exothermic because the ΔH is negative.
Step-by-step explanation:
The standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements, with all substances in their standard states.
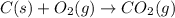
Exothermic reactions are those reactions in which heat is released and
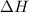 is negative.Endothermic reactions are those reactions in which heat is absorbed and
is negative.Endothermic reactions are those reactions in which heat is absorbed and
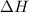 is positive.
is positive.
The reaction is exothermic as the heat is released during the formation of
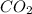 as heat of formation of
as heat of formation of
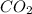 is negative.
is negative.