Answer: The number of neutron electron and proton in the nuclide
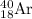 is 22, 18 and 18.
is 22, 18 and 18.
Step-by-step explanation:
Atomic number is equal to the number of protons or number of electrons for a neutral species and is specific to a particular element.
Mass number is the sum of number of protons and the number of neutrons.
Here the atomic number of Argon is 18 , thus the number of protons is 18 and thus the number of electrons is also 18.
The given mass number is 40. Thus number of neutrons = (40 - 18) = 22.
The number of neutron electron and proton in the nuclide
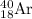 is 22, 18 and 18.
is 22, 18 and 18.