Answer: 1.
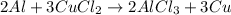
2. 3 moles of
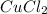 : 2 moles of
: 2 moles of
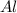
3. 0.33 moles of
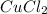 : 0.92 moles of
: 0.92 moles of
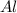
4.
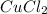 is the limiting reagent and
is the limiting reagent and
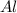 is the excess reagent.
is the excess reagent.
5. Theoretical yield of
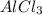 is 29.3 g
is 29.3 g
Step-by-step explanation:
To calculate the moles :
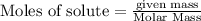
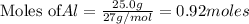
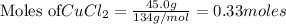
The balanced chemical equation is:
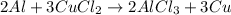
According to stoichiometry :
3 moles of
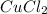 require = 2 moles of
require = 2 moles of
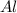
Thus 0.33 moles of
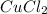 will require=
will require=
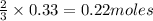 of
of
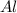
Thus
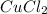 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and
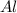 is the excess reagent.
is the excess reagent.
As 3 moles of
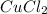 give = 2 moles of
give = 2 moles of
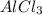
Thus 0.33 moles of
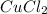 give =
give =
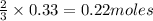 of
of
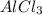
Theoretical yield of
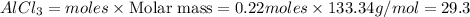
Thus 29.3 g of aluminium chloride is formed.