Answer:
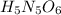
Step-by-step explanation:
Hello there!
In this case, for the determination of empirical formulas, it is firstly necessary to assume the given percentages of the constituent atoms as the masses so we can compute their moles in the formula:
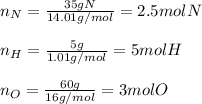
Thus, we need to divide the resulting moles, by the fewest ones (those of nitrogen) in order to determine the coefficients in the formula:
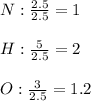
However, we need to turn all these numbers, whole numbers, so we multiply by 5 to get:
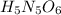
Best regards!