Answer:
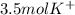
Step-by-step explanation:
Hello there!
In this case, since the mole-mole rations consider the amount of the specific ion in the whole compound, we can evidence that the potassium sulfate has two potassium ions in the molecule; in other words, there is a 1:2 mole ratio of the former to the latter; in such a way, we can perform the following proportional factor:
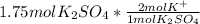
So we cancel out moles of potassium sulfate to obtain:
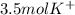
Best regards!