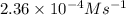Answer: The rate of the reaction is
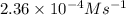
Step-by-step explanation:
Rate law says that rate of a reaction is directly proportional to the concentration of the reactants each raised to a stoichiometric coefficient determined experimentally called as order.
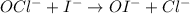
![Rate=k[OCl^-]^m[I^-]^n](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/fhsl4qnqy013puq5dzggfj.png)
where m = n = 1
![Rate=78.6M^(-1)s^(-1)* [1.14* 10^(-3)M]^1* [2.64* 10^(-3)M]^1=2.36* 10^(-4)Ms^(-1)](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/rxesp1o63tc3l51l0vyyta.png)
The rate of the reaction is