Answer
Step-by-step explanation
Given data:
8Al(s) + 3Fe₃O₄(s) ---> 4Al₂O₃(s)+ 9Fe(s)
Al₂O₃(s) ️Hf = -1669.8kJ/mol
Fe₃O₄(s) ️Hf = -1120.9 kJ/mol
Step-by-step solution:
The ️Hf for the reaction can be calculated using the formula below:
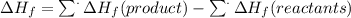
For Al(s) and Fe(s), their ΔHf is arbitrary zero.
Therefore, the ore, the ️Hf for the is:reaction
![undefined]()