Answer:
a) 3.969
b) 3.489
Step-by-step explanation:
a) Calculate the pk, value of the acid HA
PH of salt hydrolysis
P
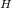 = 1/2 ( pkw + pka + logC )
= 1/2 ( pkw + pka + logC )
8.7 * 2 = 14 + log ( 0.27 ) + Pka
∴ Pka = 3.9686 ≈ 3.969
b) Calculate the PH of a solution containing 0.3 M HA and 0.1 M NaA
PH = Pka + log ( salt / acid )
= 3.9686 + log ( 0.1 / 0.3 )
= 3.9686 - 0.48 = 3.489