Answer:
1.6 cm³
Step-by-step explanation:
The density is equal to mass divided by the volume. So, we can solve the equation for the volume as follows
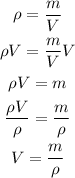
Where ρ is the density, m is the mass and V is the volume
Replacing the density by 40 g/cm³ and the mass by 64.4 g, we get
![V=(64.4g)/(40g/cm^3)=1.61\operatorname{cm}^3]()
Therefore, the answer is
1.6 cm³