Answer:
Step-by-step explanation:
Here, we know that oxygen is one of the principal component of air
As a fact, it is 21% of the total air content
What this refer to is that at any point in time, oxygen takes 0.21 the total
Mathematically, according to the Dalton's law of partialpressure, the total pressure is the sum of the individual pressure
Every constituent of the air nixture has a certain contribution in terms of the total air pressure
The partial pressure of oxygen will be the number of moles of oxygen multiplied by the total air pressure
We have this as:
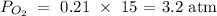
Finally, we want to know if the diver is in danger of experiencing air toxicity
We can get this from the limit. Whenever we have a partial pressure greater than 1.4 atm or greater, the toxicity sets in
Thus,we can be sure that the diver is in danger of air toxicity