Answer
10.6 liters
Step-by-step explanation
What is given:
Density of the gasoline = 0.680 g/mL
Mass of the gasoline = 7.20 kg
What to find:
To find how large of a container, (volume) in liters needed to hold the gasoline.
Step-by-step solution:
Step 1: Convert the mass of gasoline from kg to g.
Conversion factor: 1 kg = 1000 g
So 7.20 kg = (7.20 kg/1 kg) x 1000 g = 7200 g
Step 2: Determine the volume in mL using the density equation.
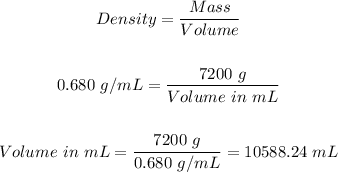
Step 3: Convert the volume in step 2 from mL to liters.
Conversion factor: 1000 mL = 1 L
So, 10588.24 mL = (10588.24 mL/1000 mL) x 1 L = 10.58824 liters
How large of a container, (volume) in liters needed to hold 7.20 kg of gasoline is approximately 10.6 liters