Answer
K₂CrO₅
Step-by-step explanation
Given:
Mass of potassium = 0.890 g
Mass of chromium = 0.590 g
Mass of oxygen = 0.727 g
What to find:
The empirical formula of the compound.
Step-by-step solution:
Step 1: Find the number of moles for each element by dividing the mass present with the relative mass of the atom.
The mole formula is given by:
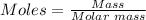
Molar mass of K = 39.098 g/mol
Molar mass of Cr = 51.996 g/mol
Molar mass of O = 15.999 g/mol
So,
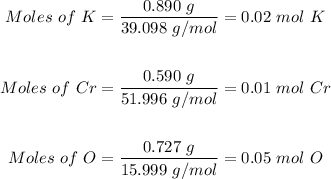
Step 2: Divide each number of moles by the lowest one, in this case
0.01.
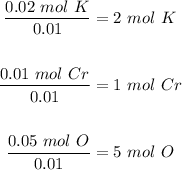
The final step is to use the mole ratio in step 2 to write the empirical formula of the compound.
The empirical formula of the compound is:
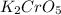
.