The question requires us to calculate the mass necessary to prepare a lead(II) nitrate (Pb(NO3)2) solution, given the volume of the solution and its molar concentration.
The following information was provided by the question:
salt used = Pb(NO3)2
volume of solution = V = 750.0 mL = 0.7500 L
molar concentration of solution = C = 0.20 mol/L
To solve this problem, we'll use two important definitions and their equations: the molar concentration and number of moles.
The molar concentration (C, given in mol/l) is defined as the number of moles of a compound (n, given in moles) divided by the volume of the solution (V, given in liters):
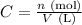
On the other hand, the number of moles of a substance (n, given in moles) can be calculated by dividing the mass of this substance (m, given in grams) by its molar mass (M, given in g/mol):
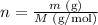
If we replace n as given by the second equation in the first equation, we'll have:
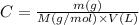
And, rearranging this last equation, we find an expression to calculate the mass of a substance from the molar concentration of a solution, its volume and the molar mass of the substance:

(where m is the mass, in g, C is the molar concentration, in mol/L, M is the molar mass, in g/mol, and V is the volume, in L).
Since the question provided the values for C and V, we'll only need the molar mass of Pb(NO3)2.
The atomic masses of Pb, N and O are 207.2 u, 14.01 u and 15.99 u, respectively. Thus, the molar mass of Pb(NO3)2 can be calculated as:
molar mass Pb(NO3)2 = (1 * 207.2) + (2 * 14.01) + (6 * 15.99) = 331.2 g/mol
Next, we apply the values of to the equation to calculate the mass of Pb(NO3)2:
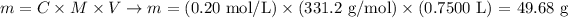
Therefore, the mass of Pb(NO3)2 required to prepare 750.0 mL of a 0.20 M solution is 49.68 g.