Answer
Formulas: Ag₂O (silver oxide), oxygen (O₂ ), and elemental silver (Ag)
Equation with labeled phases
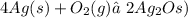
Procedure
Silver oxide can be produced by allowing silver to react with oxygen at temperatures lower than its decomposition point (195 °C).
The chemical formula for silver oxide (product) is
Ag₂O
Where the reactants are molecular oxygen (O₂ )and elemental silver (Ag).
The chemical equation with labels will be
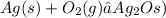
The final balanced equation is
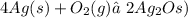
where you will find 4 silver atoms and 2 oxygen atoms in both reagents and products sides of the chemical equation.