Let's start with calculating the concentration of the fluoride ion with the provided data:
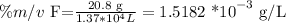
To convert to ppm, we have to remember the formula:
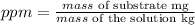
But, as the dissolvent is water and we can assume that the density is 1 g/ml, the same as 1 kg/L, we can calculate the ppm by dividing the mg of F- into the provided volume of water:
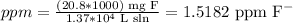
So, the answer is 1.5182 ppm F-.
For the second part, we only have to notice that ppm is the same (in this case) that mg/L. So, it means that the level is acceptable as it is 1.5182 mg/L, which is lower than 2 mg/L.