Step 1 - Finding the stoichiometry of the reaction
The given reaction is:
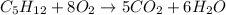
To find the stoichiometry of the reaction in moles, we just have to "read" the bigger numbers that come before the formulas of the substances:
1 mole of C5H12 reacts with 8 moles of O2 thus producing 5 moles of CO2 and 6 moles of H2O
Since the exercise is specifically asking us about the relation between C5H12 and CO2, we can simplify this statement to:
1 mole of C5H12 produces 5 moles of CO2
Step 2 - Converting this relation to a relation in grams
To convert moles to grams, we just need to multiply the number of moles by the molar mass of the substance.
Since the exercise is giving us the mass of C5H12, it would be interesting to convert its number of moles to mass (its molar mass is 72 g/mol):
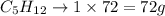
We can now rewrite the statement in step 1 as:
72g of C5H12 produce 5 moles of CO2
Step 3 - Finding how many moles of CO2 will be formed
To find the moles of CO2 that will be formed, we can set the following proportion:
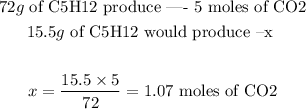
Step 4 - Converting moles to volume
The molar volume of a gas in STP conditions (standard pressure and volume) is 22.4 L/mol, which means that each mole of gas occupies 1 L. Since we have formed 1.07 moles of CO2, we have:
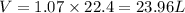
The volume of CO2 that would be formed is thus 23.96L.