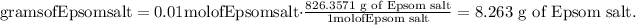1) Convert molecules of water into moles of water.
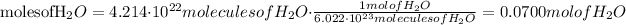
2) Use the moles of water in step 1 to find out the number of moles of Epsom salt
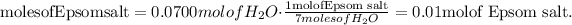
3) Find the molecular mass of one mol of Epsom salt.
Mg: 24.30 g/mol
S: 32.065 g/mol
O: 15.999 g/mol
H: 1.008 g/mol
The molecular mass of Epsom salt (MgSO4 * 7H2O) is
(24.30*1)+(32.065*1)+(15.999*4)+(1.008*14) +(15.999*7) =826.3571 g/mol
4) Convert moles of Epsom salt into grams of Epsom salt.