To solve this problem we have to use the rule of dilutions:
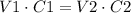
Where V1 is the initial volume, C1 is the initial concentration, V2 is the final volume and C2 is the final concentration.
In this case, we need to find V1. Solve the equation for V1 and then replace C1 for 3.75M, V2 for 190mL and C2 for 0.300M:
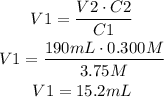
It means that the volume required is 15.2mL.