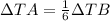The absorbed or released by a system is given by the following formula:
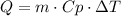
Where Q is the heat absorbed or released, m is the mass of the system, Cp is the specific heat and ΔT is the change in the temperature.
Let mA and mB be the mass of objects A and B, let CpA and CpB be the specific heats of objects A and B and let ΔTA and ΔTB be the specific heats of objects A and B. We will have that:
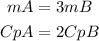
If the same amount of heat is applied to both object, we will have that:
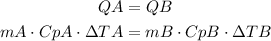
Replace mA and CpA for their equivalences in terms of mB and CpB:
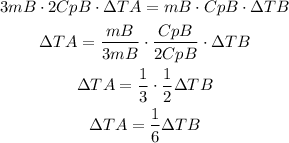
It means that the change in temperature of A is 1/6 of the change of temperature of B.