Answer
x = 2, y = 2
Step-by-step explanation
The empirical formula is the simplest whole number ration that defines constituent atoms in a species.
The molecular formula is always a multiple of the empirical formula.
Since the empirical formula of the compound i given to be CH, ithen its molecula formula is:
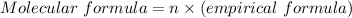
From the periodic table, the molar masses of (H = 1.00784, C = 12.011)
Thus, the molecular formula is
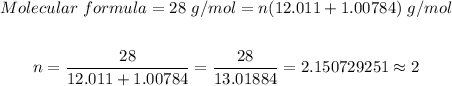
(CH)n = (CH)₂ = C₂H₂
Therefore, CxHy = C₂H₂. The value of x and y is 2 and 2 respectively.
The value of x and y if its empirical formula is CH is x = 2, y = 2