To solve this problem, we could use the ICE table as follows:
First of all, the reaction that we're working with is:
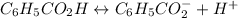
Now, let's make the table:
As you can notice, the initial concentration of C6H5CO2H is 0.536002M, and as the products haven't been formed yet, their concentration is 0.
When the change happen, the concentration of the reactant will decay "x" and the concentration of the products will increase "x". (That's the reason of the signs).
And finally, the equilibrium.
We also know that:
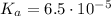
And, this equilibrium constant comes from the following:
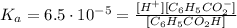
Where all concentrations are the equilibrium concentrations.
If we replace, we got that:
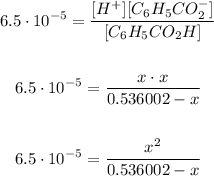
We're going to solve this equation for x:
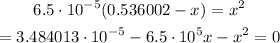
And, using the quadratic formula we obtain that the value of x is 0.000587014 approximately.
Now, if x=0.000587014, that means that [H+] = 0.000587014
And finally, remember that the pH of a solution is defined as:
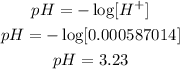
Therefore, the pH is 3.23.