Answer:
Vf = 0.0108 m³
Step-by-step explanation:
Assuming there is no change in internal energy, we can calculate the changed volume by the following formula:
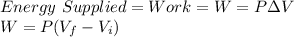
where,
W = Work = Energy Supplied = 1000 J
P = Pressure = 101325 Pa
Vf = Final Volume = ?
Vi = Initial Volume = 0.001 m³
Therefore,
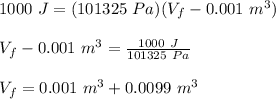
Vf = 0.0108 m³