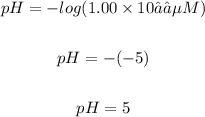Answer
A solution with pH of 5.
Step-by-step explanation
Given:
[H₃O⁺] = 1.00 x 10⁻⁵ M
To know the solution that has a hydronium ion concentration, [H₃O⁺] of 1.00 x 10⁻⁵ M, you need to calculate the pH of the solution using the formula given below:
![pH=-log\left[H₃O⁺\right]](https://img.qammunity.org/2023/formulas/chemistry/college/5y7gy0jk42b73bjhzpirqicl4twtvmjx82.png)
Put [H₃O⁺] = 1.00 x 10⁻⁵ M into the pH formula:fof