We need to find how much will be left after 6 half-lives of a radioactive isotope starting with 130g.
One way to write the amount N of radioactive isotope left after a time t, with an initial amount N₀ and a half-life τ is:
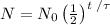
Notice that when t = τ, we have:
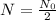
In this problem, we have:
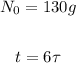
Then, we obtain:
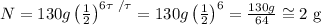
Therefore, rounding to the nearest gram, the answer is 2 grams.