Answer:
0.284L
Explanations
The balanced chemical reaction between sodium and water is expressed as:
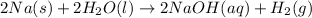
Determine the moles of sodium Na that reacted
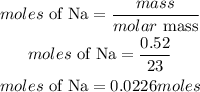
Based on stoichiometry, 2moles of sodium produces 1 mole of hydrogen gas. The moles of hydrogen gas required is given as:
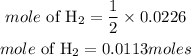
According to the ideal gas equation
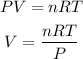
Given the following
P = 740mmHg = 0.974atm
T = 25 +273 = 298K
R = 0.08205 Latm/molK (Gas constant)
Substitute the given parameters into the formula to have:
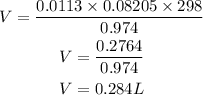
Hence the volume of hydrogen gas that evolved from the reaction is 0.284L