ANSWER
The molar concentration of NaOH is 0.0705 mol/L
Explanation:
Given the balanced equation below
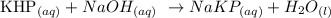
According to the balanced equation, 1 mole of KHP gives 1 mole of NaOH
Given parameters
Molar mass of KHP = 204.2 grams/mol
To find the mole of KHP, we will need to find the average grams of KHP used
• For flask 1; 0.55g of KHP was used
,
• For flask 2; 0.56g of KHP was used
,
• For flask 3; 0.56g of KHP was used
The average mass of KHP used can be calculated below using the average formula

The average mass of KHP used is 0.3886grams

The mole of KHP is 0.0019 mole
PART B
According to the balanced equation, the stoichiometry ratio of KHP to NaOH is 1: 1
Let the mole of NaOH be x
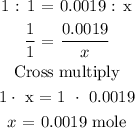
Hence, the mole of NaOH is 0.0019 mole
PART C
Given the following parameters
0. The volume of NaOH used in flask 1 = 26.70mL
,
1. The volume of NaOH used in flask 2= 27.09mL
,
2. The volume of NaOH used in flask 3 = 26.96mL
The next step is to convert the mL to L
For flask 1
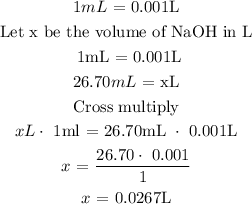
Using the same conversion process
The volume of NaOH in L in flask 2 = 0.02709L
The volume of NaOH in L in flask 3 = 0.02696L
Hence, the molar concentration of the solution in each flask can be calculated as follows
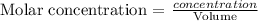
For flask 1
Mole of NaOH = 0.0019 mole
Volume of NaOH = 0.0267L

For flask 2
Mole = 0.0019 mole
Volume = 0.02709L

For flask 3
Mole = 0.019mole
Volume = 0.02696 L
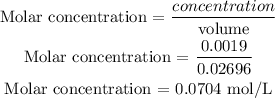
PART D
Average molar concentration can be found using the below formula
