Answer:
M=0.380 M.
Step-by-step explanation:
Hello there!
In this case, given those two solutions of aluminum bromide and zinc bromide, it is firstly necessary to compute the moles of bromide ions in each solution as shown below:
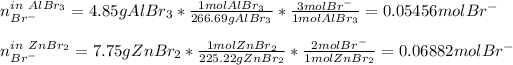
Now, we compute the total moles of bromide:
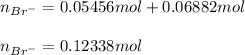
Then, the total volume in liters:
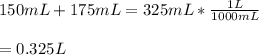
Therefore, the concentration of total bromide is:
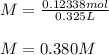
Best regards!