Answer
1.88 grams
Step-by-step explanation
Given:
3H2(g) + N2(g) ----> 2NH3(g)
Volume of NH3(g) = 3 L
What to find:
The grams of N2(g) required.
Step-by-step solution:
From this balanced equation, 3 moles of H2(g) react with 1 mole N2(g) to produce 2 moles NH3(g).
That can also mean 3 liters of H2(g) reacts with 1 liter N2(g) to produce 2 liters of NH3(g).
Thus, if it takes 1 liter of N2(g) to produce 2 liters of NH3(g),
Then it will take x liters of N2(g) to produce 3 liters of NH3(g)
To get x, cross multiply and divide both sides by 2 liters NH3(g):
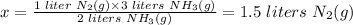
1.5 liters of N2(g) are required to produce 3 L of NH3(g).
The final step is to convert 1.5 liters of N2(g) to grams.
1 mole of N2(g) occupies 22.4 L at STP
Note: 1 mole of N2(g) = 28.01 g
Thus, if 28.01 grams N2(g) occupies 22.4 L at STP
Then the mass of N2(g) in 1.5 L of N2(g) is:
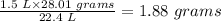
Therefore, the grams of N2(g) required to 3L of NH3(g) is 1.88 grams