The reaction we have is 1.839 g of cacium hydroxde (Ca(OH)₂) with 25.0 mL of solution of 0.200 M sulfuric acid (H₂SO₄).
Let's start with the reaction:
We have an acid and an base, so this is a neutralization reaction:
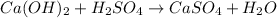
Notice that the Ca²⁺ and SO₄²⁻ are alredy balanced.
But we have 2 OH⁻ and 2 H⁺ on the left side to form only one H₂) on the right side, so we must add a 2 to get the balanced chemical equation:
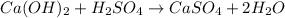
As said before, the type of the reaction is f. Neutralization Reaction.
Now, to get how much of each we will get if all the limitant reacts, we first need to know how many moles we have of each.
We have 1.839 g of Ca(OH)₂, and its molar weight is approximately 74.093 g/mol, so we have:
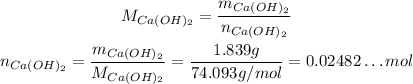
For the H₂SO₄, we have 25.0 mL, which is the same as:
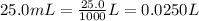
And the solution is 0.200 M = 0.200 mol/L, so:
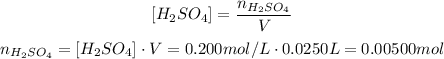
From the equation, we see that Ca(OH)₂ and H₂SO₄ react 1 to 1. Since we have less of H₂SO₄, this is the limitant reactant.
So, all 0.00500 mol of H₂SO₄ will react, so we will end up with no H₂SO₄, 0 g of H₂SO₄.
From the 0.02482...mol of Ca(OH)₂, 0.00500 mol will react, remaining:
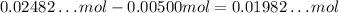
Covnerting it to grams, we have:
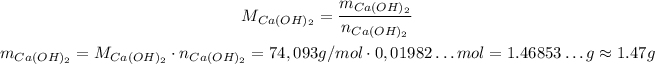
So, in the end we will have approximately 1.47 g of Ca(OH)₂.
For the products, let's start with CaSO₄, we have also 1 to 1 with respect to the reactants, so we will have 0.00500 mol, in the end. Using its molar weight of approximately 136.141 g/mol, we have the mass:
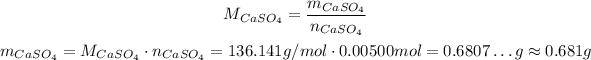
So, in the end we will have approximately 0.681g of CaSO₄.
For water, we already have water in the solution. But considering only the water we will produce from the reaction, we have a ratio of 1 reactant for 2 waters, so we will produce twice the 0.00500 mol, that is, 0.0100 mol.
Using the molar weight of water of approximately 18.015 g/mol, we have:
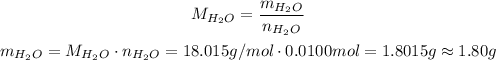
So, in the end we will have approximately 1.80g of water produced.
All in one, we will have:
Grams of calcium hydroxide: 1.47g
Grams of sulfuric acid: 0g
Grams of water: 1.80g
Grams of calcium sulfate: 0.681g