INFORMATION:
We need to find how many kilojoules of energy are needed to convert 280 grams of water at -20 C° to water at 75 C°
STEP BY STEP EXPLANATION:
Since the temperature of water in all process goes from -20°C to 75°C, we would have a change in the state when temperature is equal to 0°C.
So, we must divide the problem in three parts:
1. Temperature from -20°C to 0°C:
In this part, we need to use the next formula
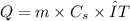
Where, m represent the mass, Cs is the specific heat capacity and ΔT is the temperature change.
In this case,
m = 280 grams = 0.28 Kg
Cs = 2100 J/Kg°C
ΔT = 0°C - (-20 °C) = 20°C
Now, replacing in the formula
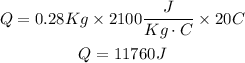
2. Change in the state from ice to water:
In this part, we need to use the next formula
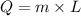
Where, m represents the mass and L is the specific latent heat.
In this case,
m = 280 grams = 0.28 Kg
L = 334 J/Kg
Now, replacing in the formula
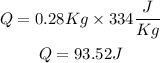
3. Temperature from 0°C to 75°C:
In this part, we need to use the formula from the first part.
In this case,
m = 280 grams = 0.28 Kg
Cs = 4186 J/Kg°C
ΔT = 75°C - 0 °C = 75°C
Now, replacing in the formula
![\begin{gathered} Q=0.28Kg*4186\frac{J}{Kg\operatorname{\degree}C}*75\operatorname{\degree}C \\ Q=87906J \end{gathered}]()
Finally, we must add up the three parts and then convert it to kilojoules.
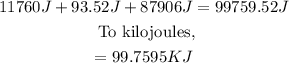
ANSWER:
99.7595 kilojoules of energy are needed to convert 280 grams of water at -20 C° to water at 75 C°