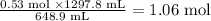Answer
1.06 mol
Step-by-step explanation
A beaker contains 0.53 mol of potassium bromide in 648.9 mL of water
An additional 648.9 mL of water is added implies the volume of the water will become (648+648)mL = 1297.8 mL
Therefore, the number of moles of potassium bromide in 1297.8 mL will be: