The first step to answer this question is to balance the given equation:
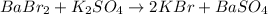
According to this, one mole of BaBr2 produces 2 moles of KBr. Use this information to find how many moles of KBr will be produced from 17.3 moles of BaBr2:
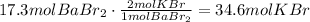
It means that the correct answer is the first choice a. 34.6.