ANSWER
The final volume of the gas is 67.1 mL
Step-by-step explanation
Given that;
The initial volume of the gas is 60.0mL
The initial temperature of the gas is 20 degrees Celcius
The initial pressure of the gas is 770 mmHg
The final temperature of the gas is 40 degrees Celcius
The final pressure of the gas is 735 mmHg
Follow the steps below to find the final volume of the gas
Step 1; Write the general gas law equation
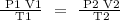
Step 2; Convert the temperature to degrees kelvin
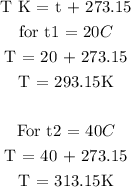
Step 3; Substitute the given data into the formula

Therefore, the final volume of the gas is 67.1 mL