a) We have a Fe ion with a positive charge +2, therefore we will also have a Fe ion with a +3 charge.
The charge difference is +1, this means that the Fe3+ ion gained an electron, therefore 1 must be put in front.
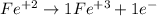
b) Now, in the second reaction we have 4 oxygens in the reactants and one in the products, so we put the coefficient 4 in front of H2O and thus we will have 4 oxygens in the products.
Now it would be necessary to balance the hydrogens, we have 8 hydrogens in the products and 1 in the reactants, so we put the coefficient of 8 in front of the hydrogen
Now the Mn, there is an atom of Mn in the reactants, the coefficient 1 is placed in front of the Mn+2.
So far the balanced reaction will go like this:
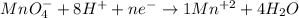
We need to balance the electrons. For that, we see what is the oxidation state of Mn in the molecule MnO4-. Oxygen has an oxidation state of -2.:
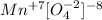
The oxidation state in the MnO4 molecule is +7, therefore it must gain 5 electrons to be left with a +2 charge.
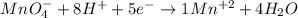
And so we have the balanced equation.