- First, to write the Electron Configuration for Pb, we need to know the total number of electrons in the Pb atom. We can take that information from the Periodic Table of Elements:
Electrons in the Pb atom = 82
- Second, we complete the orbitals with the electrons following the Möller diagram:
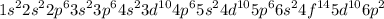
That is the complete Electron Configuration for Pb.