Given,
Mass of the iron, m=4.8 kg
Initial temperature, T₁=22 °C=295.15 K
Final temperature, T₂=400 °C=673.15 K
The specific heat capacity of the iron is, c=462 J/kg·K
The heat energy required is calculated using the formula,
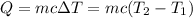
On substituting the known values,
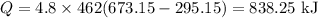
Therefore the energy required is 838.25 kJ