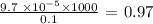Answer:
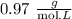
Step-by-step explanation:
Here, we want to make a conversion
Mathematically, we have to know the conversion values
1000 g = 1 kg
10 dL = 1L (this means 1 dL = 0.1 L)
So, we are going to apply these values.
We will multiply the numerator by 1000 and divide by 0.1
We have this as follows: