ppm or part er million is used to measure the concentration of solute in a given volume of solution.
We can calculate it by the formula
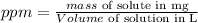
In our question we have the next data:
*Mass of methanol
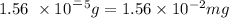
where we have to convert from g to mg.
* The volume of the solution is:
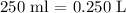
where we have to convert from ml to L
Putin the values on the right-hand side of the equations above we get:
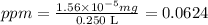
Looking at the option in the question, by similarity we chose the option c, that is , we chose ppm=0.0611