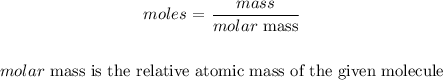Answer:
Step-by-step explanation:
Here, we want to relate moles to relative atomic mass
In terms of unit, we have moles as mol and relative atomic mass as g/mol
Thus, by definition, the relative atomic mass is the mass in grams per mole of the substance
Mathematically, we can relate the two as follows: