Answer: the percent composition of Al in Al2(SO4)3 is 15.771%, and the best option to answer the question is letter C.
Step-by-step explanation:
The question requires us to calculate the percent composition of aluminum (Al) in the compound aluminum sulfate (Al2(SO4)3).
To calculate the percent composition of an element in a compound, such as Al in Al2(SO4)3, first we need to calculate the total molar mass of the compound, then calculate the molar mass that corresponds to the element in the compound, and then estabilish the relationship between these two values.
1) Calculating the molar mass of Al2(SO4)3:
The atomic masses of Al, S and O are 26.981, 32.065 and 15.999 amu, respectively. Thus, we can calculate the molar mass of Al2(SO4)3 as:
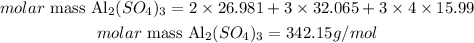
Therefore, the molar mass of Al2(SO4)3 is 342.15 g/mol.
2) Calculating the mass of Al in Al2(SO4)3:
There are 2 atoms of Al in the molecule of Al2(SO4)3, thus we can calculate how much, in g/mol, of Al is there in the molecule as:
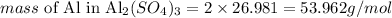
Therefore, we can say that each mol of Al2(SO4)3 contains 53.962 g of Al.
3) Calculating the percent composition of Al in Al2(SO4)3:
If we consider the molar mass of Al2(SO4)3 as 100%, we can calculate the percentage of Al in this mass as it follows:
mass of 1 mol of Al2(SO4)3 = 342.15 g ------------------------ 100%
mass of Al in 1 mol of Al2(SO4)3 = 53.962 g ---------------- x
Solving for x, we'll have:
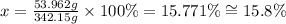
Therefore, the percent composition of Al in Al2(SO4)3 is 15.771%, and the best option to answer the question is letter C.