Step 1 - Understanding the combustion of hydrocarbons
C5H12 is a hydrocarbon, i.e., a molecule that has only C and H atoms in its composition. Whenever we talk about the combustion of hydrocarbons, there will always be two predictable products: CO2 and H2O.
Remembering that combustion means "reaction with O2", we can write this equation as:
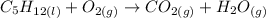
Step 2 - Balancing the equation
Let's take a look at how many atoms there are in each side of this equation:
Right hand side: 5 C atoms, 12 H atoms and 2 O atoms
Left hand side: 1 C atom, 2 H atoms and 3 O atoms
We can begin to balance it by multiplying CO2 by 5:
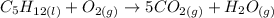
Now let's multiply H2O by 6 in order to get 12 H in the LHS:
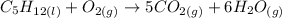
Finally, note there are now 16 O in the LHS. Let's multiply O2 by 8 then:
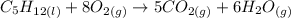
The equation is now properly balanced.