The question requires us to write down the chemical equation for the combustion of methane, where 4.32 ng of this compound burns in excess oxygen. The question also requires us to calculate the amount of reactants and products at the end of the reaction and to classify the reaction.
When methane burns in the presence of oxygen, the combustion reaction of methane occurs and its equation can be written as:
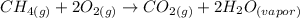
This reaction is classified as a combustion reaction (letter g).
Since the amount of methane was provided by the question (4.32 ng) and the question mentions excess of oxygen, we can assume methane as the limiting reactant and use it to calculate the amount of products obtained.
First, we need to convert the mass given into the number of moles of methane using its molar mass. We'll also need the molar mass of CO2 and H2O further to answer the question completely.
The atomic masses that we'll use are:
C = 12.01 u
H = 1.01 u
O = 15.99 u
We calculate the molar masses considering the atomic mass and the number of atoms of each element in the molecules:
molar mass (CH4) = (1 * 12.01) + (4 * 1.01) = 16.05 g/mol
molar mass (CO2) = (1 * 12.01) + (2 * 15.99) = 43.99 g/mol
molar mass (H2O) = (2 * 1.01) + (1 * 15.99) = 18.01 g/mol
Now that we have the molar masses, we can use the molar mass of CH4 to calculate how many moles are there in 4.32 ng of this compound. Note that the mass is given in ng, which corresponds to 10^-9 g:
16.05 g CH4 -------------------- 1 mol
4.32 x 10^-9 g CH4 ---------- x
Solving for x, we have that 4.32 ng of CH4 corresponds to 2.69 x 10^-10 moles of CH4.
Next, we'll use the amount of moles of CH4 we calculated and the stoichiometric relation given by the chemical equation to calculate how many moles of CO2 and H2O would be produced:
1 mol CH4 -------------------------- 1 mol CO2
2.69 x 10^-10 mol CH4 -------- y
Solving for y, we have that 2.69 x 10^-10 moles of CO2 would be obtained from 4.32 ng of CH4.
1 mol CH4 -------------------------- 2 mol H2O
2.69 x 10^-10 mol CH4 -------- z
Solving for z, we have that 5.38 x 10^-10 moles of H2O would be obtained from 4.32 ng of CH4.
At last, we use the molar masses of CO2 and H2O, calculated previously, to convert the number of moles of these substances into their masses:
1 mol CO2 ------------------------------ 43.99 g CO2
2.69 x 10^-10 mol CO2 ------------ a
Solving for a, we have that the mass produced of CO2 is 1.18 x 10^-8 g or 11.8 ng.
1 mol H2O ------------------------------ 18.01 g H2O
5.38 x 10^-10 mol CO2 ------------ b
Solving for b, we have that the mass produced of H2O is 9.69 x 10^-9 g or 9.69 ng.
To summarize the answer, we can write:
- The balanced chemical reaction: CH4 + 2O2 -> CO2 + 2H2O
- The type of reaction: combustion reaction
- At the completion of reaction, we would have:
- Grams of methane: 0 g, since methane is the limiting reactant, it would be completely consumed.
- Grams of oxygen: it is not possible to affirm the amount of oxygen because the initial amount wasn't provided.
- Grams of carbon dioxide: 1.18 x 10^-8 g or 11.8 ng of carbon dioxide are produced.
- Grams of water vapor: 9.69 x 10^-9 g or 9.69 ng of water vapor are produced