The balanced reaction to produce lithium oxide is:
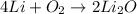
To produce 2 moles of lithium oxide, 4 moles of lithium and 1 mole of oxygen are needed.
The first step to solve these questions is to convert the mass of lithium to moles using its molecular weight (MW=29.88g/mol):
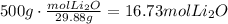
a. Use the ratio of moles of lithium to moles of lithium oxide to find how many moles of lithium are needed to produce 16.73moles of lithium oxide:
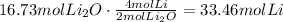
Convert this to mass using lithium molecular weight:
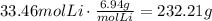
232.21 grams of Lithium are needed.
b. Follow the same procedure to find the moles of O2 needed:
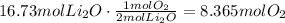
At this point we can use the ideal gas law to find the volume occupied by 8.365 moles of O2. Use STP (P=1atm, T=273.15K) and R as 0.082atmL/molK:
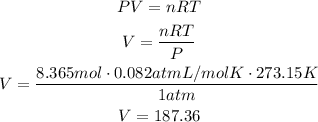
It means that 187.36L of O2 are needed.