Answer: Mass of solute needed = 107.74 grams of CuSO4
Explanation
GIVEN
• Volume ,=, 900 ml = 900/10,00 = 0.9L
,
• Concentration, = 0.75 M = 0.75 mol/L
,
• Molar mass CuSO4, = 159,609 g/mol
(i) Calculate moles of CuSO4

Therefore , Moles CuSO4 = 0.675
(ii) Calculate Mass of CuSO4
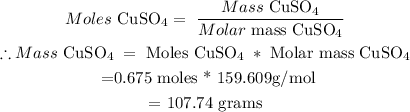
Therefore; Mass of solute needed = 107.74 grams of CuSO4