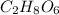Answer:
Step-by-step explanation:
Molecular formula can be calculated by finding the ratio of the molecular molar mass to the empirical molar mass, then multiplying the subscripts of the empirical molar mass by that ratio.
The empirical molar mass is 3(12.01) + 4(1.01) + 3(16.00) = 88.07
The molecular molar mass is 180g
180/88.07 = 2
So the molecular formula is