Answer: 2.125 moles of NaClO4 would be produced if 4.250 moles of O2 reacted completely with NaCl.
Step-by-step explanation:
The question requires us to calculate the amount of moles of sodium chlorate (NaClO4) that would be produced when 4.250 moles of oxygen (O2) react completely with sodium chloride (NaCl).
The unbalanced chemical equation for the reaction between O2 and NaCl to form NaClO4 can be written as:
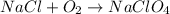
Note that the equation above is not balanced: we need to adjust the amount of O atoms.
There are 2 O atoms on the left side, while there are 4 O atoms on the right side, thus we can adjust the coefficient of O2 from 1 to 2. The balanced chemical equation can be written as:
![NaCl+2O_2\operatorname{\rightarrow}NaClO_4]()
From the balanced chemical equation, we can see that 2 moles of O2 are necessary to produce 1 mol of NaClO4. With this information, we can calculate how many moles of NaClO4 would be obtained from 4.250 moles of O2:
2 mol O2 --------------------------- 1 mol NaClO4
4.250 mol O2 -------------------- x
Solving for x, we'll have:
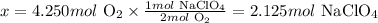
Therefore, 2.125 moles of NaClO4 would be produced if 4.250 moles of O2 reacted completely with NaCl.