We just need to use the density formula
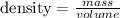
They give the mass = 9.18 g
And Volume is obtained by the increment in volume = 12.14ml - 9.00ml = 3.14ml
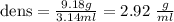
Specific gravity is just to express the density in relation to the density of water since the density of water is 1g/ml