Answer
basic (alkaline)
Step-by-step explanation
To know if the solution is acidic, basic or neutral, you need to determine the pH of the solution.
![pH=-log[OH^+]](https://img.qammunity.org/2023/formulas/chemistry/college/l9sdpc7xfdgxoalq2w1vegnonjtw3dl07o.png)
Putting [OH⁺] = 1.00 x 10⁻¹⁰ into the pH formula, we have
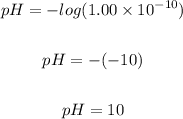
Note that the pH scale ranges from 0 to 14, with 7 being neutral. pHs less than 7 are acidic while pHs greater than 7 are alkaline (basic).
Therefore, the solution is basic (alkaline)