Let H₁ be the concentration of a solution with a pH of 1, and H₂ be the concentration of a solution with a pH of 2, then:
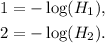
Then:
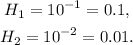
Therefore:
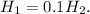
Therefore the H concentration in a solution with a pH of 2 is 0.1 times of a solution with a pH of 1.
Answer: Second option.